Objectives
At the end of this chapter you should:
have a basic understanding of some deterioration processes;
have a visual reference for some of the issues discussed in other sections; and
have a basic understanding of some of the problems you may encounter in your collection.
Introduction
Deterioration is caused by physical damage and chemical activity—usually in combination. For many materials, physical damage can create conditions that are favourable for chemical activity.
For example, as aluminium corrodes, an aluminium oxide layer forms on the surface which protects the rest of the metal from corrosion. If this layer is scratched or broken in any way, un-oxidised aluminium will be exposed and it will corrode. Fortunately the corrosion produces a new oxide layer which protects the rest of the metal.
Iron objects are often coated to protect them from contact with moisture and oxygen. If they are not protected they rust. Rust is iron oxide; but unlike aluminium oxide it does not protect the underlying metal from further corrosion. If a coating applied to an iron object is scratched or broken in any way, the object rusts. At first, the rust is localised, but it spreads gradually over the whole object, destroying it totally.
Chemical activity often accelerates physical damage, or leaves objects more susceptible to physical damage. For example, pressure-sensitive adhesives—as used to make sticky tapes—age and become less sticky. The adhesives harden and no longer hold things together. This also happens to adhesives such as rubber cement. Collages and other items which include a lot of adhesives can fall apart once the adhesives have aged.
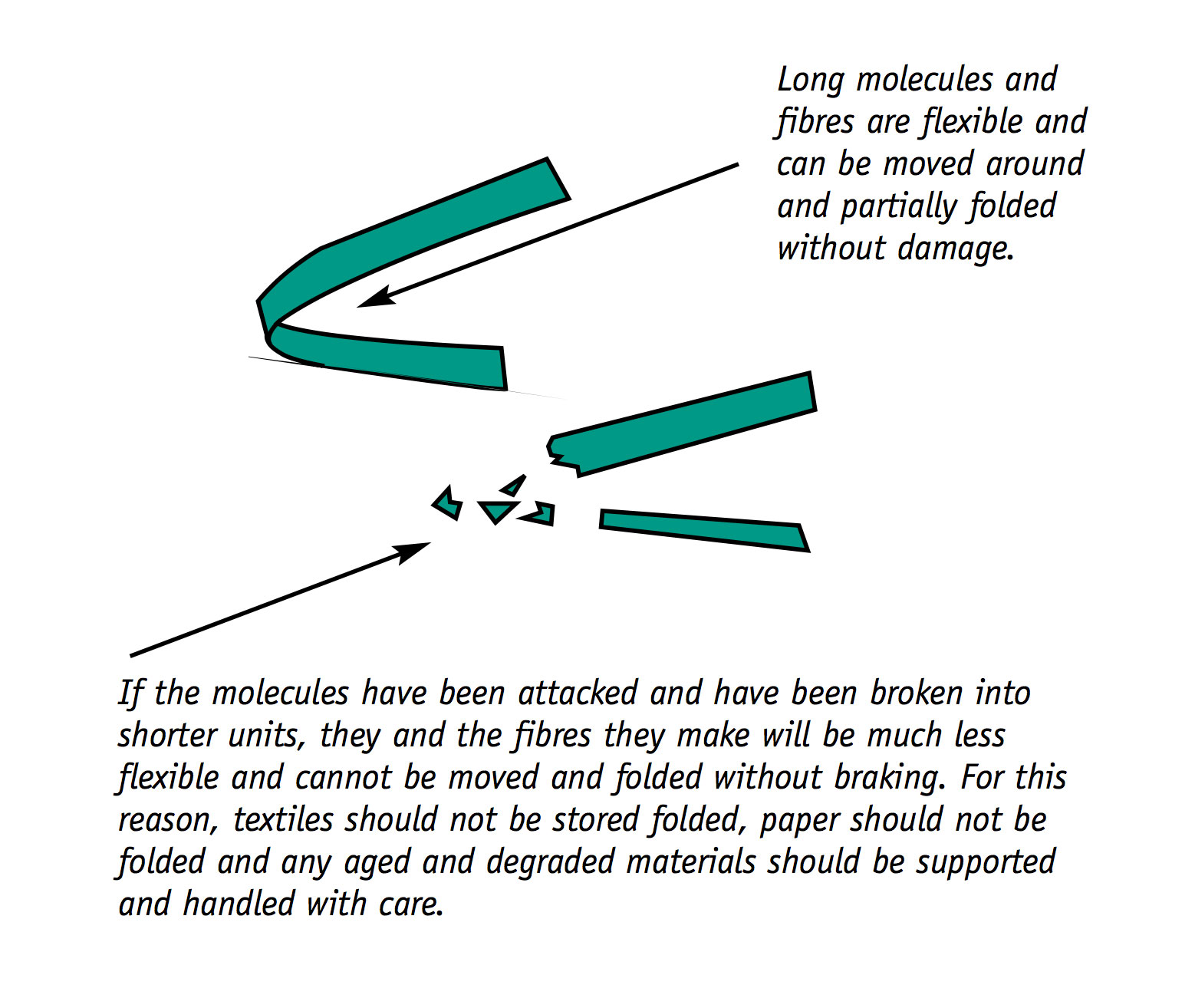
Paper that was once flexible and easy to use can become brittle, to the point where it crumbles away to fragments when handled roughly. This extreme vulnerability to physical damage is a result of chemical deterioration. Acids within the paper attack the paper’s fibres, making them shorter and much less flexible.
Examples of deterioration
The following examples illustrate the common changes that occur in materials as they deteriorate chemically:
flexible organic materials, for example, paper, fabrics and some plastics, often become brittle;
the change in solubility characteristics and loss of flexibility of some adhesives, paint layers, varnishes and coatings;
colour change, for example, dyes fading and becoming discoloured; and
corrosion of metals.
Flexible organic materials becoming brittle
Paper is made up of cellulose fibres. Fabrics are made up of cellulose, protein or man-made fibres.
All of these fibres are made up of long ribbon-like molecules. Flexible plastic films are also made up of long molecules.
The shortening of the molecules has a major impact on the physical characteristics of the materials.
Change in solubility characteristics and loss of flexibility
Many paints, inks, varnishes and coatings dry by a combination of evaporation and chemical change. The chemical change that takes place is called crosslinking. The relative importance of evaporation and chemical change in the drying stage depends on the original formulation of the paint, ink or varnish.
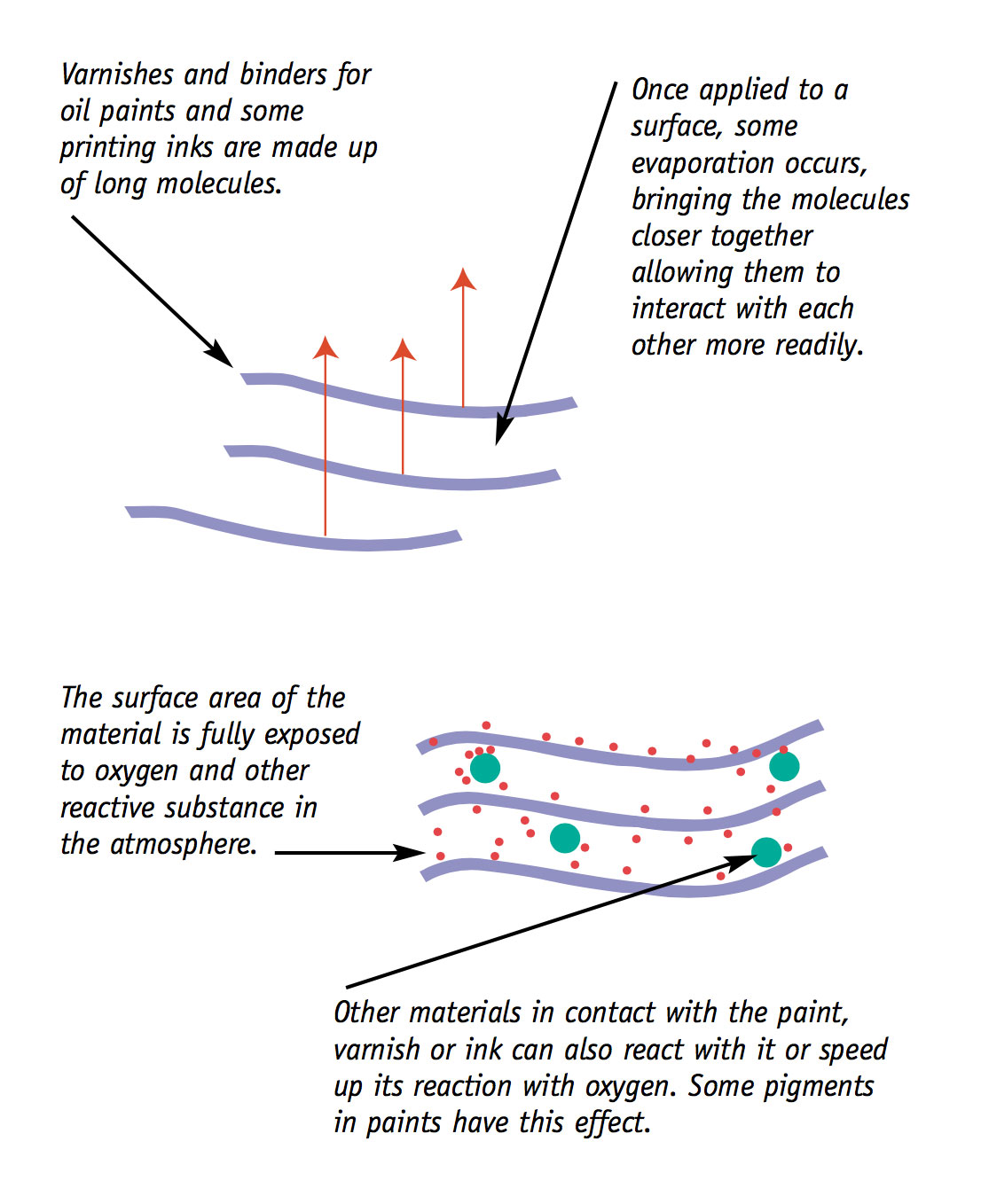
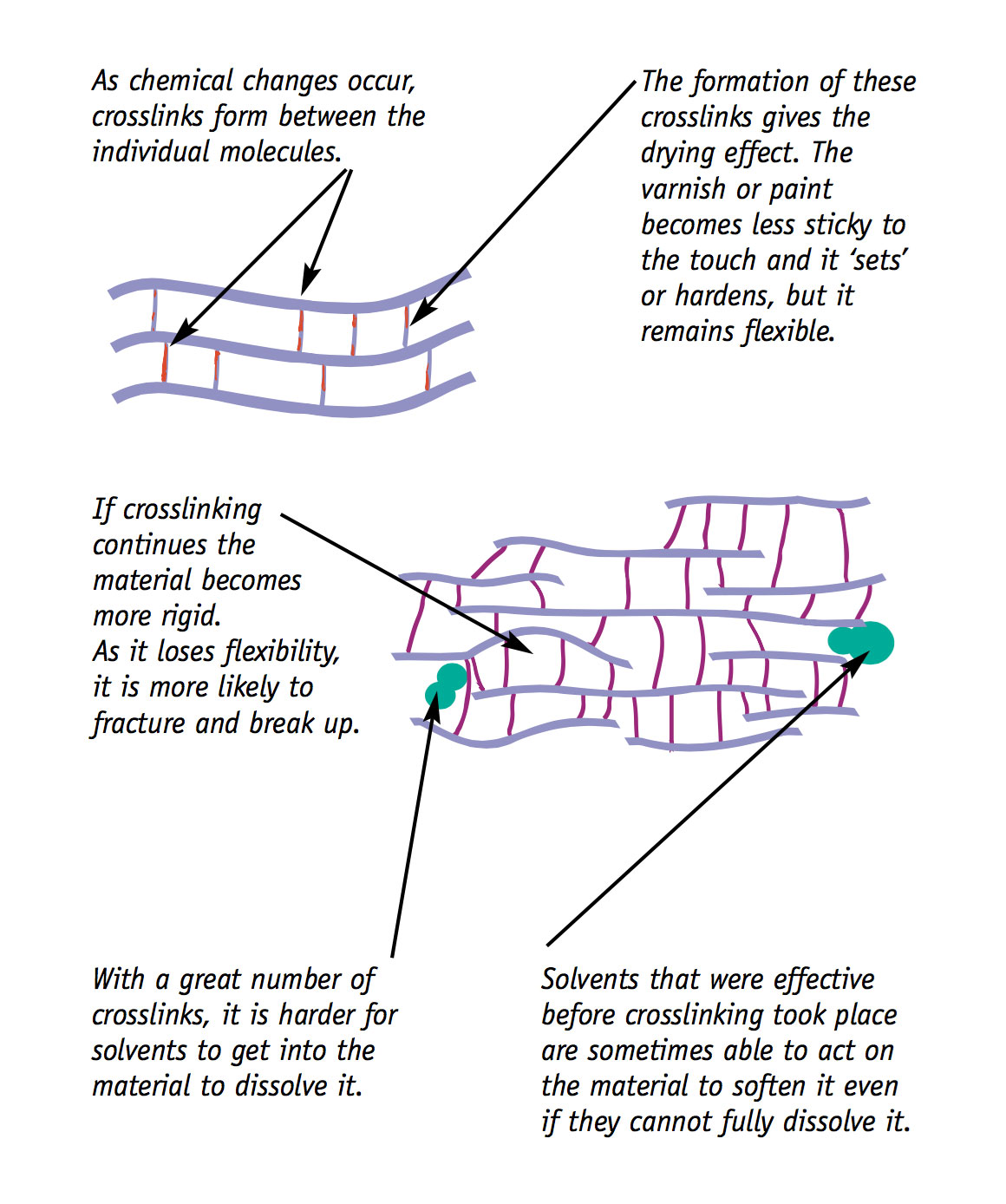
When it is part of the drying process, crosslinking is seen as a useful and desirable chemical reaction. But it is not desirable when it is seen as an ageing process which causes:
- sticky tape adhesives to set and become insoluble;
- varnishes to become less soluble and to discolour; and
- paint and ink films to become very hard and brittle.
It is important to note that crosslinking can occur at the same time as other parts of the same molecules are being broken into smaller units.
Colour change
We see materials as coloured because they selectively absorb some wavelengths of visible light and reflect others.
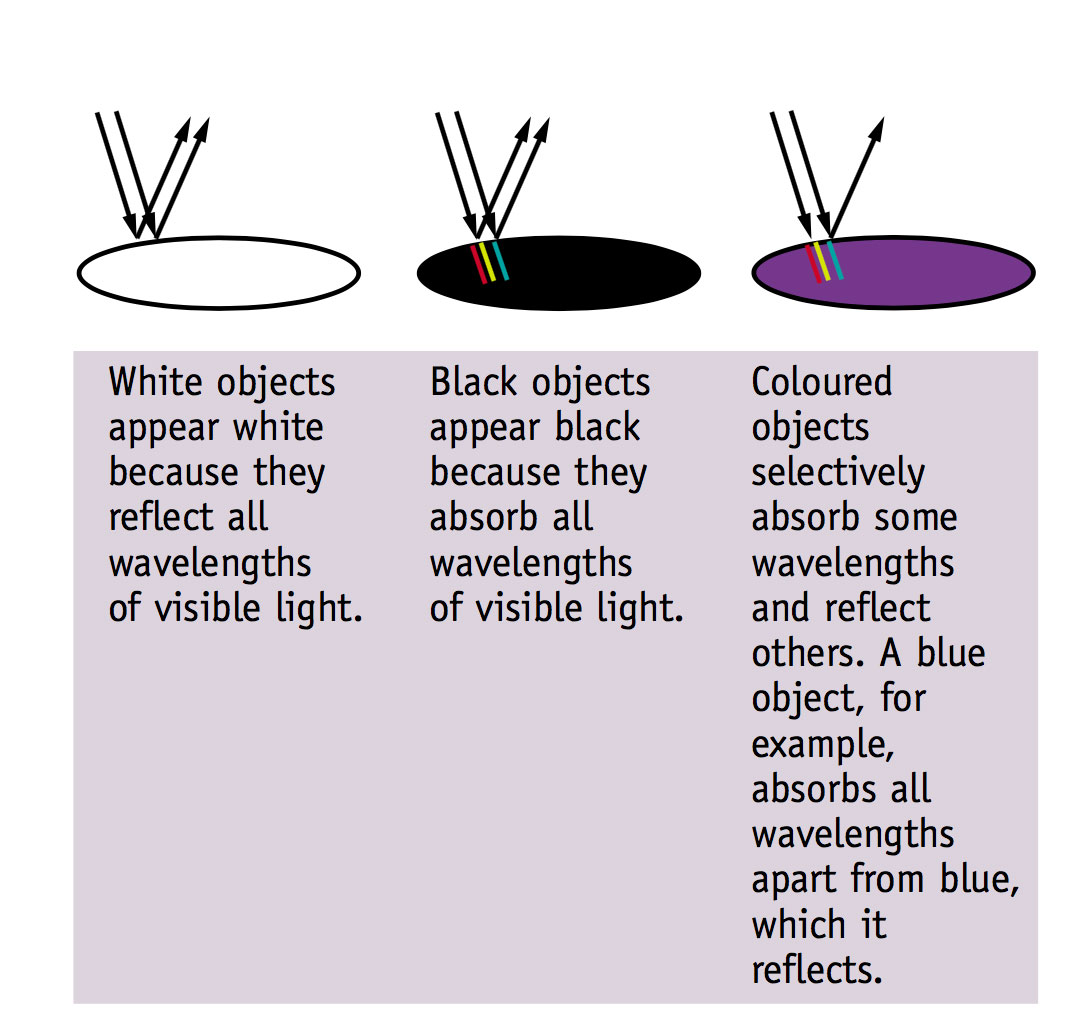
At a molecular level there are certain chemical groups which determine the absorption of particular wavelengths of light—these groups are called chromophores and are responsible for the colour of materials.
In organic materials, colour is associated with particular molecular structures that absorb and emit visible light of specific frequencies; that is, chromophores are groupings of atoms within a molecule.
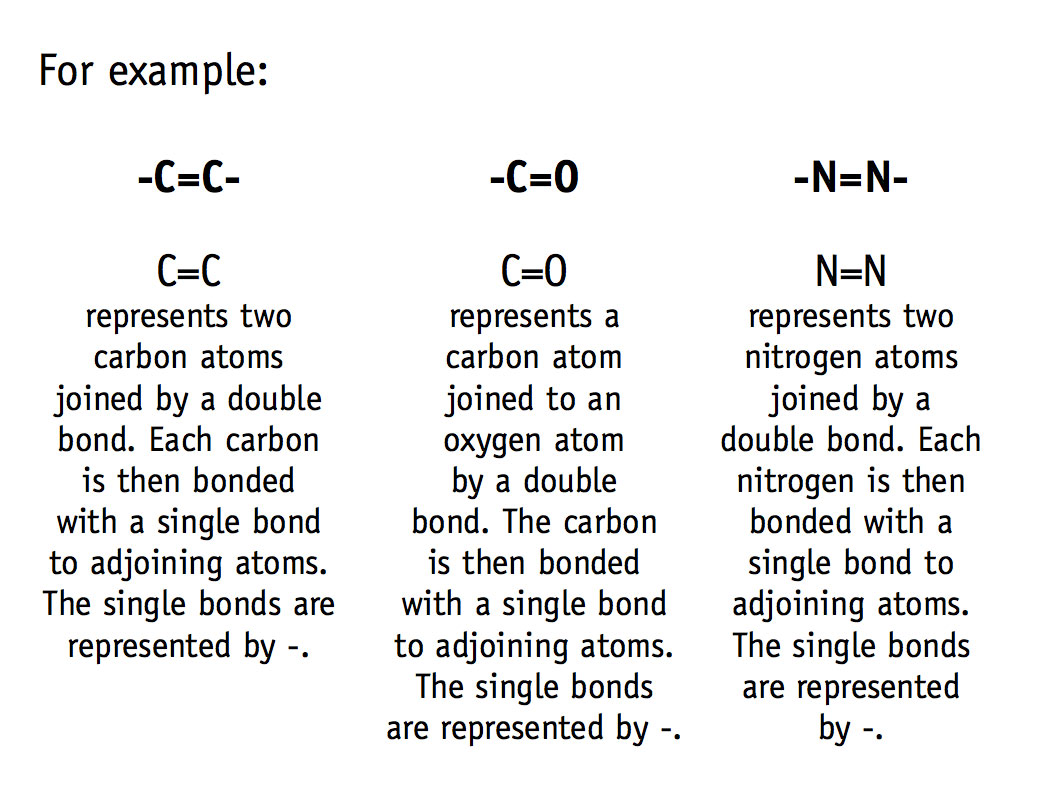
Double bonds are strong, but they are far more reactive than single bonds, so they are broken readily during chemical reactions.
When talking about flexible materials becoming brittle, and the change in solubility characteristics and loss of flexibility of materials, we examined the breaking and crosslinking of large organic molecules. The chromophores described above occur in these molecules.
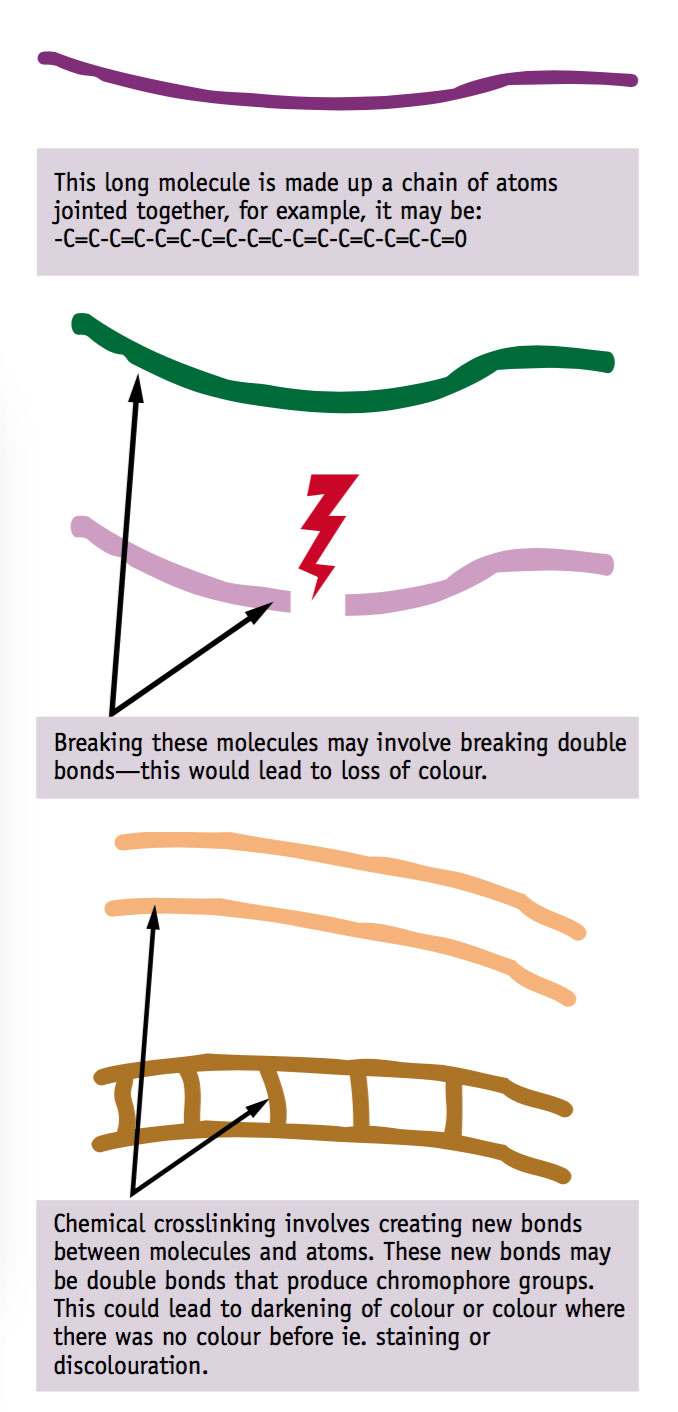
If molecules are being broken down and crosslinked at the same time, a whole range of colour changes are possible. Just what the colour changes will be is difficult to predict.
Corrosion of metals
The chemical deterioration of metals is known as corrosion.
Some metals are protected by a layer of corrosion. For example, when aluminium is exposed to air, it corrodes to form aluminium oxide which covers the surface of the metal item.
Other metals like iron are not protected by their corrosion products.
Some metal objects are electroplated. Electroplating is used to make cheaper metals look like silver. The physical properties of the materials are dominated by the underlying metal.
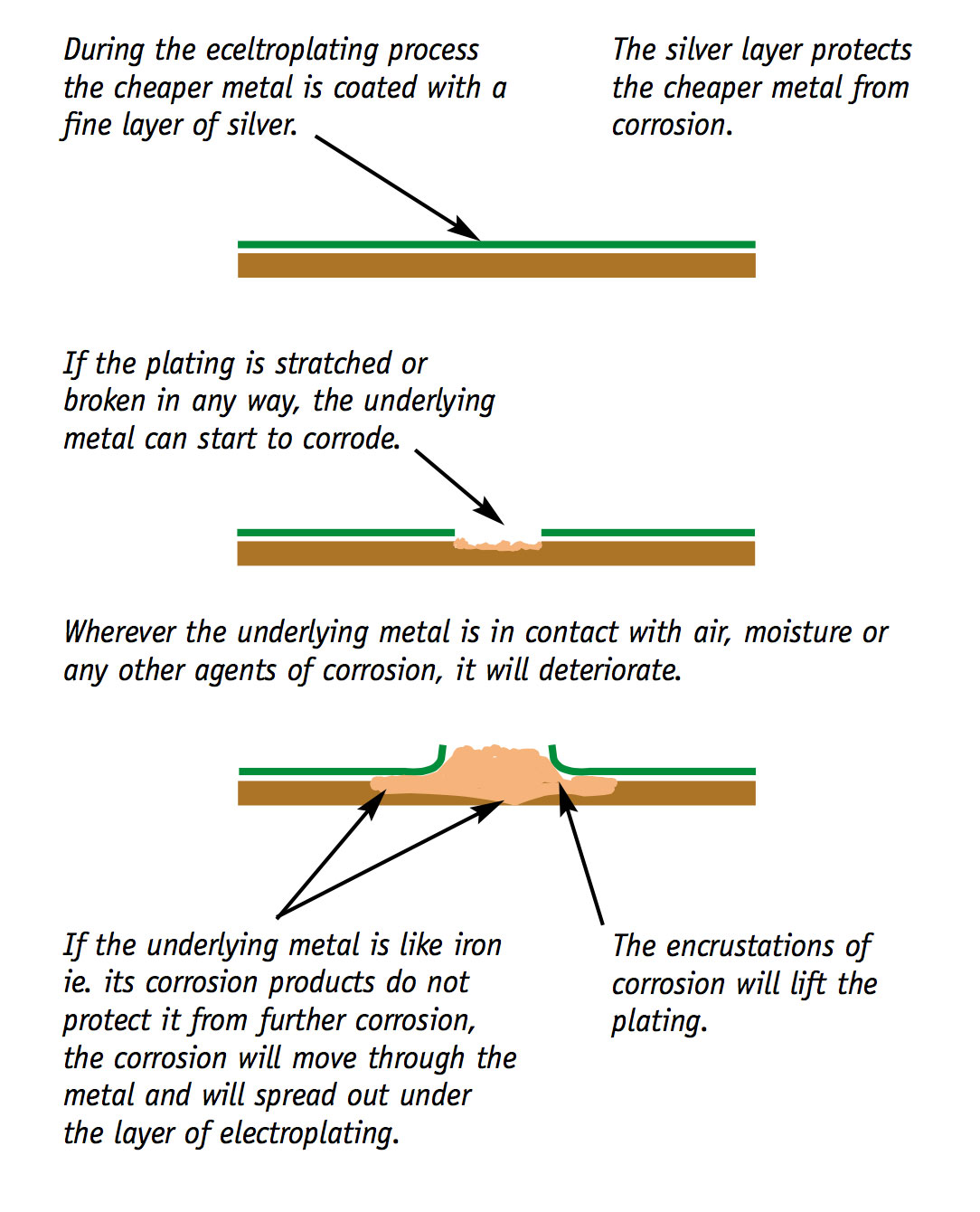
As stated earlier, this section provides a simplified overview of some of the processes of deterioration. For further information about the effects of these processes and on minimising these effects, please refer to the chapters relating to specific types of materials.
For further reading
Crafts Council Conservation Science Teaching Series 1982, Science for Conservators Book 1— An Introduction to Materials, Crafts Council, London.
Crafts Council Conservation Science Teaching Series 1983, Science for Conservators Book 2— Cleaning, Book 2, Crafts Council, London.
Mills John S. and White Raymond, 1987, The Organic Chemistry of Museum Objects, Butterworths, London.
Self-evaluation quiz
Question 1.
Which of the following statements are true?
a) For many materials, physical damage can create conditions which are favourable for chemical activity.
b) Physical damage and chemical deterioration are in no way linked.
c) Chemical deterioration can accelerate physical damage.
d) None of the above.
Question 2.
Crosslinking:
a) is part of the drying process of oil paints, some printing inks, and varnishes;
b) is part of the ageing process that makes some adhesives and varnishes less soluble over time;
c) can lead to discolouration of some adhesives;
d) is all of the above.
Question 3.
Which of the following statements are false?
a) Crosslinking and breaking of molecules can occur simultaneously.
b) The change in the length of organic molecules can have a major impact on the physical characteristics of materials.
c) All metals are protected from further corrosion by their corrosion products.
d) Chemical deterioration has no effect on the colour of materials.
Answers to self-evaluation quiz
Question 1.
Answer: a) and c) are true.
Question 2
Answer: d).
Question 3
Answer: c) and d) are false. Aluminium is an example of a metal that is protected by its corrosion products but many other metals are not. Aluminium will not be protected if the corrosion layer is broken. The breaking and re-forming of bonds in molecules, processes which are part of chemical deterioration, can have significant effects on colour changes, both fading and staining. Chemical deterioration causes these changes.